
Look at the illustration of the carbon atom again.
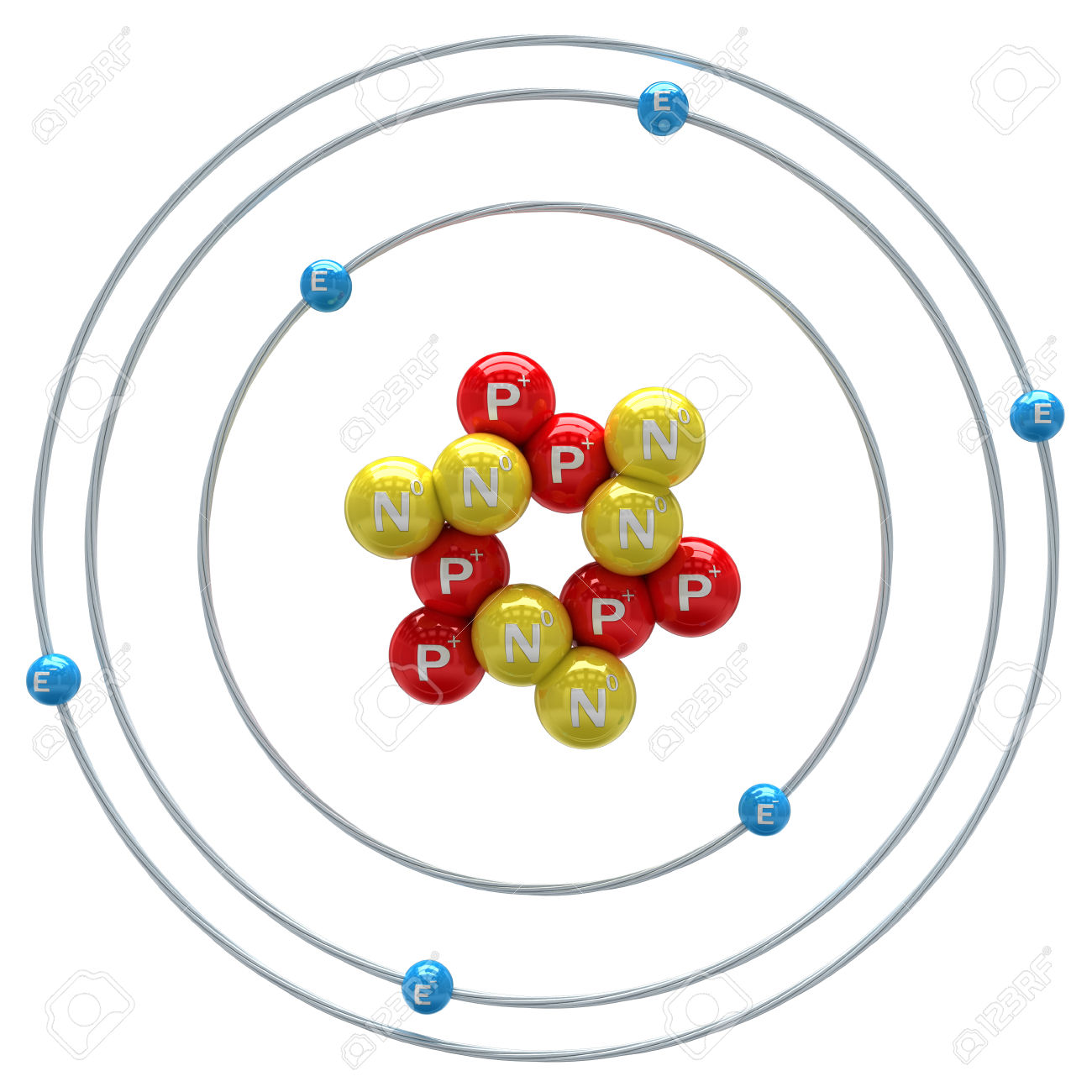
Atom of carbon bohr model plus#
In writing the electron configuration for carbon the first two electrons will go in the 1s orbital. For carbon, the atomic number is 6, and the atomic mass number is 12 (6 protons plus 6 neutrons).

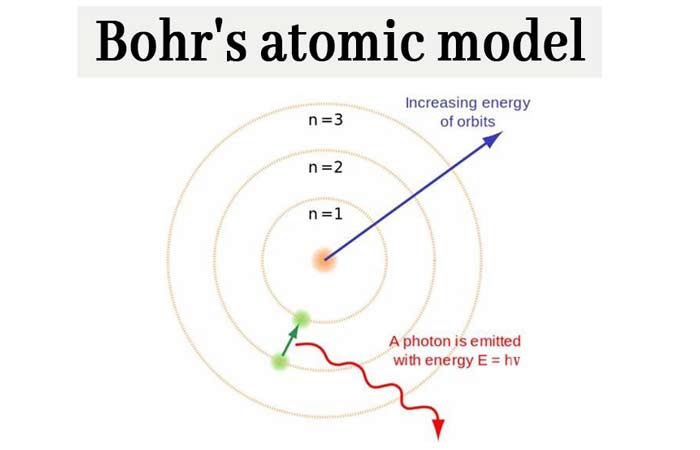
\) is the Rydberg constant in terms of energy, Z is the atom is the atomic number, and n is a positive integer corresponding to the number assigned to the orbit, with n = 1 corresponding to the orbit closest to the nucleus. Carbon is the sixth element with a total of 6 electrons. Bohr’s Model of an Atom Bohr’s model consists of a small nucleus (positively charged) surrounded by negative electrons moving around the nucleus in orbits.


 0 kommentar(er)
0 kommentar(er)
